An ideal OSD Point-of-Care test should be:
- Objective
- Quantitative
- Rapid (less than 15 min)
- Specific
- Reproducible
- Informing/guiding clinical management and/or therapeutic decisions
- Simple to allow implementation by ancillary staff with an efficient workflow
Tear-based Point-of-Care (T-POC) quantitative results support early diagnosis and intervention as well as therapeutic monitoring to confirm adequate disease control or progression.

-Zaina Al-Mohtaseb, MD
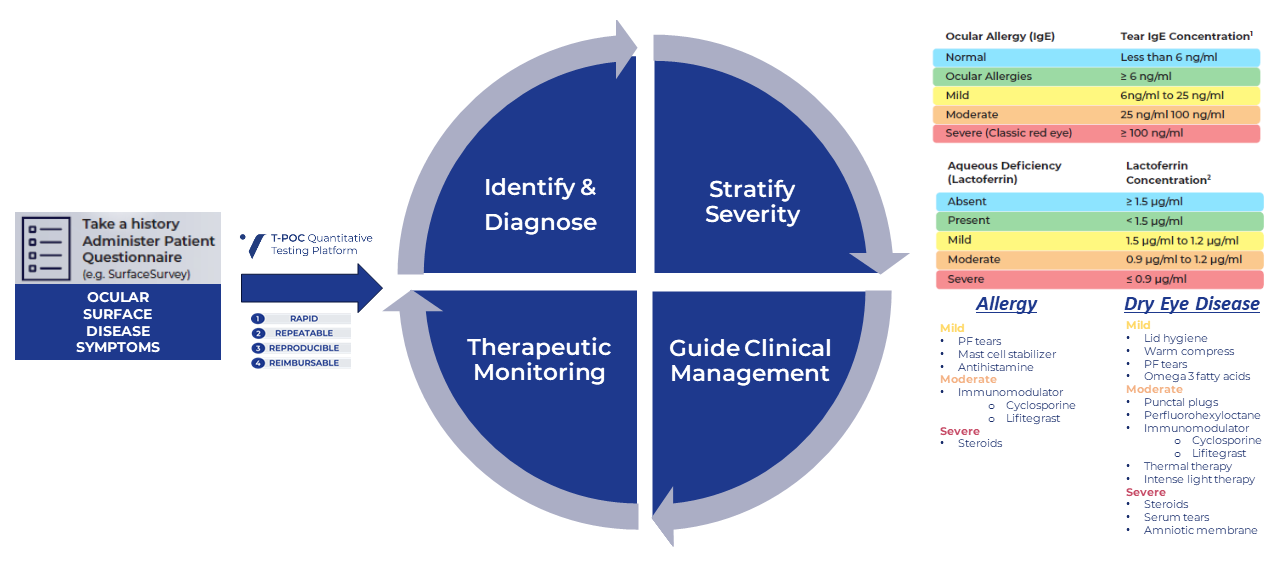
4 Phases of Precision Eyecare Starts with T-POC Quantitative Tear Testing
Click to Enlarge Image
TEAR-BASED POINT-OF-CARE (T-POC) QUANTITATIVE TESTING PLATFORM
The T-POC Quantitative Testing Platform, exclusively from Verséa Ophthalmics, is being used to improve patient care in the clinical setting for dry eye disease, ocular allergies, acute conjunctivitis, keratoconus, perioperative management of cataract and refractive surgery and contact lens fittings. The tests are simple to perform, accurate, rapid, repeatable, reproducible, and reimbursable.
Ocular surface disease patients, including Dry Eye Disease sufferers, can be diagnosed, treated, and monitored directly within your practice using quantitative IgE and lactoferrin results delivered within minutes.
Answer questions such as:
- What is the cause?
- Is it aqueous-deficient or evaporative disease?
- Is there an allergic component?
- Is the patient’s therapeutic regimen sufficient?
T-POC LACTOFERRIN TESTING

Click to Enlarge Image
- Normal lactoferrin levels = 2.11±0.74⁴
- Significant decrease in lactoferrin concentrations occurs in dry eye disease (DED)³
- Low Lactoferrin levels <1.4 mg/mL directly correlates to DED caused by aqueous deficiency³
- Severity of DED can be determined by the Lactoferrin level³
- Lactoferrin ≤ 0.9 mg/ml has an 83% sensitivity and 98% specificity for dry eye disease and a 95% specificity for Sjogren’s disease
- Low levels of lactoferrin indicate suppressed lacrimal function – aqueous deficiency³
- Resistant to reflex tearing³
- Low Lactoferrin levels indicate DED with increased surgical risk
- Low Lactoferrin levels may indicate the cause of contact lens intolerance²⁻⁴
- Risk of contact lens dehydration, leading to discomfort
- High levels of lactoferrin indicate elevated tear proteins, which could lead to excessive contact lens deposits, causing visual disruption, contact lens discomfort
- Changes in Lactoferrin levels may show the efficacy of the prescribed treatment⁵
- Lactoferrin levels are normal, and not reduced, in the setting of meibomitis-related rosacea¹

Click to Enlarge Image
T-POC TOTAL IgE Testing
Ocular allergy is a common IgE mediated immunological inflammatory process of the anterior surface of the eye that includes seasonal/intermittent, perennial/persistent, vernal, and atopic keratoconjunctivitis²

Click to Enlarge Image
- IgE testing can help differentiate ocular allergy (OA) from dry eye disease (DED) and infectious conjunctivitis³
- Elevated IgE causes tear film instability³
- Total IgE in the tears of patients who improved within treatment for 7 days was significantly lower than that in the tears of the patients who improved after treatment for 7 days
- Total IgE concentration in tears of patients with recurrent illness was significantly higher than that in patients without recurrent disease⁴
- Tear IgE correlated with the speed of improvement and recurrence of ocular allergic disease⁴
- Changes in IgE levels may show the efficacy of prescribed treatment⁴
- Tear IgE is a modifiable risk factor for keratoconus (KCN) progression
- High IgE is predictive of keratoconus cross linking failures

Click to Enlarge Image
An Ideal POC Test
Click to Enlarge Image
Click to Enlarge Image
T-POC TESTING IS BILLABLE USING THE FOLLOWING UNIQUE CPT CODES:

To learn more, visit our Resources page
Clinical Algorithms: Where Does T-POC Testing Fit?

Click to Enlarge Image
1. O’Brien TP, Jeng BH, McDonald M, Raizman MB. Curr Med Res Opin 2009;25(8):7953-1967. 2. Lin H, Yiu SC. Dry eye disease: A review of diagnostic approaches andtreatments. Saudi J Ophthalmol. 2014 Jul;28(3):773-87. 3. Pepose JS, Wilhelmus KR Divergent approaches to the management of corneal ulcers. Am J Ophthalmol. 7992;774:630-632. 4. Focus on Dry Eye Professional. Prevalence of Chronic Dry Eye. http://www.focusondryeye.com/_professionals/_pro_CDE_info/CDE_prevalence.htm. Accessed July 77,2017. 5. Sheppard JD. Dry eye moves beyond palliative therapy. Ma nag Care. 2003;72(suppl):6-8. 6. Thomas Chester, Sum it (Sam) Garg, Josh Johnston, Brandon Ayers & PreeyaGupta (2023) How Can We Best Diagnose Severity Levels of Dry Eye Disease: Current Perspectives, Clinical Ophthalmology, 77:, 7S87-7604, DOI: 70.2747/OPTH.5388289. 7.Nomura K, Takamura E. Tear lgE concentrations in allergic conjunctivitis. Eye (Lond). 7998;72 ( Pt 2):296-8.
2. Monzón S, Arrondo E, Bartra J, Torres F, Basagaña M, Del Mar San Miguel M, Alonso R, Cisteró-Bahima A. Conjunctivitis and Total IgE in Lacrimal Fluid: Lacrytest Screening. J Allergy (Cairo). 2009;2009:518903. 2] ] Bielory L, Delgado L, Katelaris CH, Leonardi A, Rosario N, Vichyanoud P. ICON: Diagnosis and management of allergic conjunctivitis. Ann Allergy Asthma Immunol. 2020 Feb;124(2):118-134
3. Chester T, Garg SS, Johnston J, Ayers B, Gupta P. How Can We Best Diagnose Severity Levels of Dry Eye Disease: Current Perspectives. Clin Ophthalmol. 2023 Jun 5;17:1587-1604.
4. Versura P, Bavelloni A, Grillini M, Fresina M, Campos EC. Diagnostic performance of a tear protein panel in early dry eye. Mol Vis. 2013 Jun 6;19:1247-57. 2] ] Chester T, Garg SS, Johnston J, Ayres B, Gupta P. How Can We Best Diagnose Severity Levels of Dry Eye Disease: Current Perspectives. Clin Ophthalmol. 2023 Jun 5;17:1587-1604. 3] Achtsidis, V., Kozanidou, E., Bournas, P., et al. Dry Eye and Clinical Disease of Tear Film, Diagnosis and Management. US ophthalmic review.2014; 07:109.
5. Bao J, Tian L, Meng Y, Wu B, Wang J, He J, Shao Q, Wang C, Jie Y, Zhang L. Total IgE in tears accurately reflects the severity and predicts the prognosis of seasonal allergic conjunctivitis. Clin Transl Allergy. 2022 Mar;12(3):e12139.
6. Ahuja P, Dadachanji Z, Shetty R, Nagarajan SA, Khamar P, Sethu S, D’Souza S. Relevance of IgE, allergy and eye rubbing in the pathogenesis and management of Keratoconus. Indian J Ophthalmol. 2020 Oct;68(10):2067-2074.
Verséa is a registered trademark of Verséa Health, Inc.
©2024 Verséa Ophthalmics, Inc. All rights reserved.

If you would like to learn more about our Tear-based Point-of-Care (POC) Quantitative Testing Platform, please contact us at ophthalmics@versea.com
Subscribe to the Verséa Newsletter
Subscribe to the Verséa Newsletter

© 2024 Verséa Health, Inc. All rights reserved.
